Clinical Trials in Health / Pharma, pre- and post-market
Pre-clinical and clinical trials performed at our investigation sites and/or in healthcare facilities.
ISO 9001 certified, Eurofins Pharmascan has been a recognized player in the pharmaceutical and dermo-cosmetics industry for more than 20 years. We have built our reputation on the quality of our services, as demonstrated by published studies and satisfied customer audits.
Our teams offer standard, innovative or tailor-made protocols to assess the safety/tolerance and efficacy/performance of health-oriented products.

Pre-clinical trials
In Healthcare Facilities
In our Clinical Trial Centers
Support in all stages of your clinical trials
Our various departments ensure that your tests are carried out in accordance
with local regulations, GCP-ICH, ISO 14155 and the various applicable standards.
Eurofins Pharmascan offers you a turnkey service, for solutions that are always more adapted to the requirements of your projects. Our project managers ensure the overall management of all phases of your tests:
We evaluate all your healthcare-related pharmaceutical products and medical devices.
The pharma and health products

Panels of healthy subjects & patients
The EUROFIN panels involve several subjects in France and worldwide from a wide range of demographic categories (infants, children, adolescents, adults and the elderly).
Varied fields of investigation




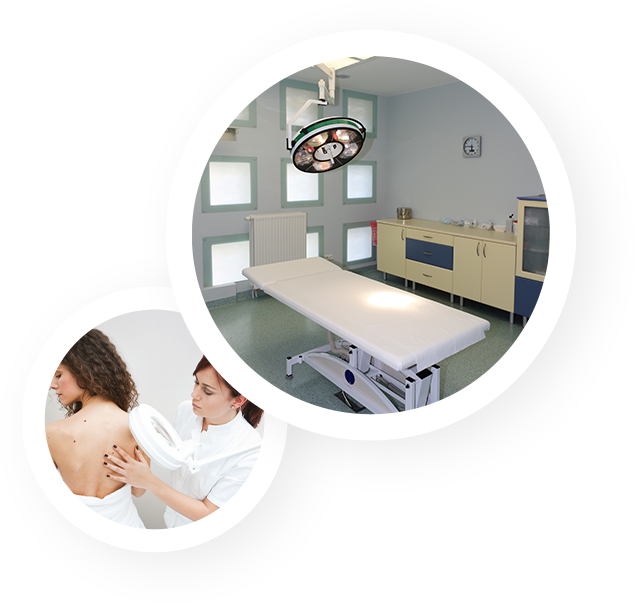
A team of specialists dedicated to clinical research
and high-tech premises
Our services at your disposal:
- Team of Project Managers and CRA
- Regulatory Affairs
- Scheduling
- eCRF design
- Subcontracting and feasibility
- Recruitment
- Medical department: salaried and/or temporary doctors providing daily medical assistance, pharmacists
- Technical department: clinical research technicians, nurses
- Biostatistics
- Quality
- Clinical logistics: product service, instrumentation, purchasing
- R&D
- Scientific communication
- IT
Our accredited, state-of-the-art laboratories in France, Poland, Tunisia, Thailand and Mauritius are fully equipped with high-performance, high-tech instruments to provide objective, reliable and quality data.
Easy to access for those participating in the studies, Eurofins Pharmascan laboratories meet the safety and comfort standards required to carry out your studies: rooms dedicated to clinical evaluations and storage of investigation products, equipment dedicated to the conservation of biological samples, air conditioning, temperature and humidity control probes, etc